QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
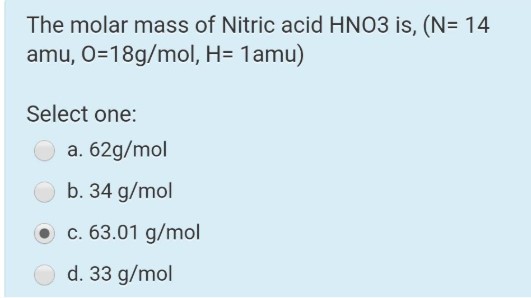
The molar mass of Nitric acid HNO^3 is, (N= 14 amu, O= 18g/mol, H= 1amu)
Select one:
a. $62 \mathrm{~g} / \mathrm{mol}$
b. $34 \mathrm{~g} / \mathrm{mol}$
c. $63.01 \mathrm{~g} / \mathrm{mol}$
d. $33 \mathrm{~g} / \mathrm{mol}$
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the molar mass of each element in HNO$_1$
The molar mass of Nitrogen (N) is given as 14 amu. The molar mass of Oxygen (O) is given as 18 g/mol. The molar mass of Hydrogen (H) is given as 1 amu. However, we need to convert the molar mass of Hydrogen from amu to g/mol to maintain consistency. We know that 1 amu is approximately equal to 1 g/mol. So, the molar mass of Hydrogen (H) is 1 g/mol.
Step 2: Calculate the molar mass of HNO$_1$
Molar mass of HNO$_3$ = (1 g/mol) + (14 g/mol) + 3 $\times$ (18 g/mol)
Final Answer
The molar mass of Nitric acid HNO$_1$ is 69 g/mol (option c).
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students