QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
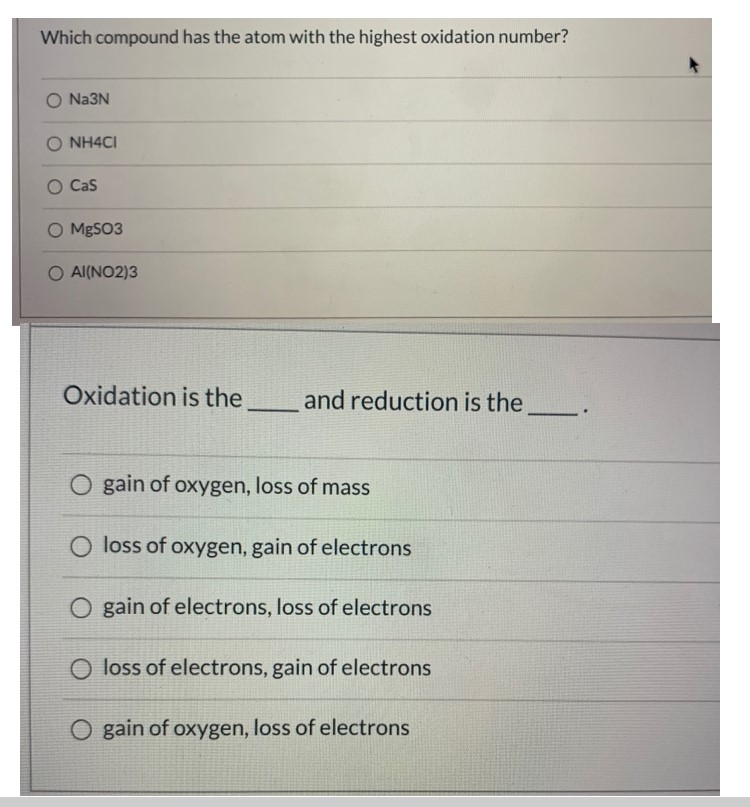
Which compound has the atom with the highest oxidation number?
- ☐ Na^3N
- ☐ NH^4Cl
- ☐ CaS
- ☐ MgSO^3
- ☐ Al(2$)3
Oxidation is the ______ and reduction is the ______.
- ☐ gain of oxygen, loss of mass
- ☐ loss of oxygen, gain of electrons
- ☐ gain of electrons, loss of electrons
- ☐ loss of electrons, gain of electrons
- ☐ gain of oxygen, loss of electrons
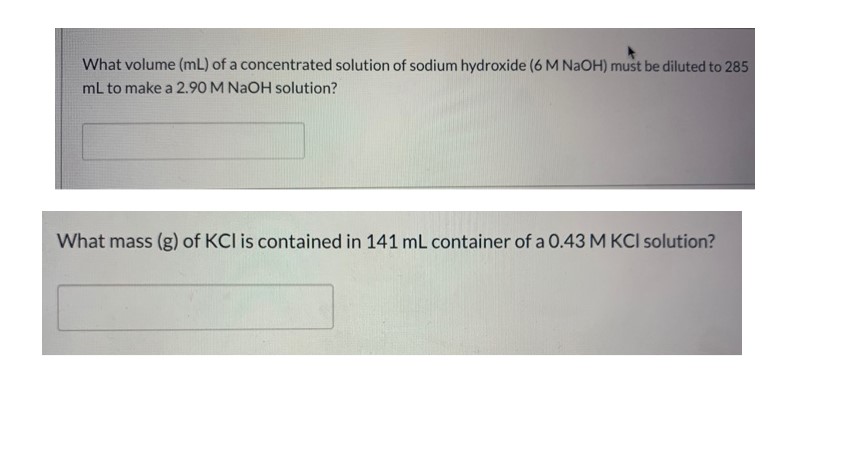
What volume (mL) of a concentrated solution of sodium hydroxide ( 6 M NaOH ) must be diluted to 285 mL to make a 2.90 M NaOH solution?
What mass (g) of KCl is contained in 141 mL container of a 0.43 M KCl solution?
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the compound with the atom having the highest oxidation number.
The oxidation number is the charge that an atom has when it forms a chemical bond. The atom with the highest oxidation number in the given choices is Nitrogen (N) with an oxidation number of + 5 in Ammonium Nitrite (Al(NO2)3).
Final Answer
Step 2: Understand the process of oxidation and reduction. Oxidation is the loss of electrons, while reduction is the gain of electrons. In other words, when an atom loses electrons, it is oxidized, and when an atom gains electrons, it is reduced. Step 3: Calculate the volume of a concentrated solution of sodium hydroxide (6 M NaOH) to be diluted to make a 2.85 M NaOH solution. To find the volume of the concentrated solution to be diluted, we can use the formula: M^1V^1 = M^2V^2 where: - M^1 is the initial molarity (concentration) - V^1 is the initial volume - M^2 is the final molarity (concentration) - V^2 is the final volume We are given: - M^1 = 6 M - M^2 = 2.85 M - V^2 = 285 mL We need to find V^1: V^1 = (M^2 * V2) / M^1 Substituting the given values: V^1 = (2.85 M * 285 mL) / 6 M Step 4: Perform the necessary calculations. V^1 = (810.75 M.mL) / 6 M V^1 = 135.125 mL Since we cannot have a fraction of a milliliter, we will round up to the nearest whole number: V^1 = 135 mL Step 5: Calculate the mass of KCl in a 141 mL container of a 0.43 M KCl solution. First, we need to find the number of moles of KCl in the solution. We can use the formula: n = c * V where: - n is the number of moles - c is the concentration - V is the volume We are given: - c = 0.43 M - V = 141 mL We need to find n: n = 0.43 M * 141 mL Step 6: Perform the necessary calculations. n = 0.43 mol/L * 0.141 L n = 0.06003 mol Now, we can find the mass of KCl using the formula: m = n * M where: - m is the mass - n is the number of moles - M is the molar mass We are given: - n = 0.06003 mol - M(KCl) = 74.55 g/mol We need to find m: m = 0.06003 mol * 74.55 g/mol Step 7: Perform the necessary calculations. m = 4.477 g
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students