QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
Which diagram is the correct electron dot diagram for magnesium?
- A.
- Mg.
- B.
- Mg.
- C.
- Mg
- D.
- Mg
Attachments
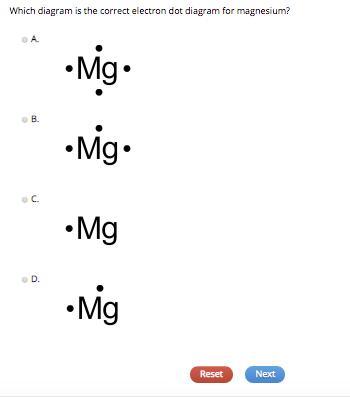
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Understand the problem
The problem asks us to identify the correct electron dot diagram for magnesium (Mg) from the given options. Electron dot diagrams, also known as Lewis structures, are a way to represent the valence electrons around an atom. Magnesium is in the second period of the periodic table and has 2 valence electrons.
Step 2: Identify the correct electron dot diagram
Let's analyze the given options: A. Mg: This option has 4 dots around magnesium, which is incorrect since magnesium has only 2 valence electrons. B. : This option has 2 dots on the right side of magnesium, which is incorrect because valence electrons should be symmetrically distributed around the atom. C. Mg: This option has 2 dots, one above and one below the magnesium atom. While this distribution is symmetric, it is not the standard way to represent electron dot diagrams. In the standard form, valence electrons are placed on the four sides (up, down, left, and right) of the atom. D. Mg: This option has 2 dots, one on each side of the magnesium atom, which is the standard and correct way to represent the electron dot diagram for magnesium with 2 valence electrons.
Final Answer
Based on our analysis, the correct electron dot diagram for magnesium is option D.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students