QQuestionChemistry
QuestionChemistry
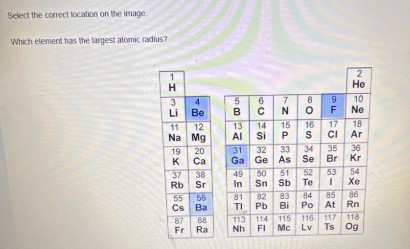
Which Element has the largest atomic radius
Select the correct location on the image.
Which element has the largest atomic radius?
| 1 | 2 |
| --- | --- |
| H | He |
| 3 | 4 |
| Li | Be |
| 11 | 12 |
| Na | Mg |
| 19 | 20 |
| K | Ca |
| 37 | 38 |
| Rb | Sr |
| 55 | 56 |
| Cs | Ba |
| 82 | 84 |
| Fr | Ra |
| 5 | 6 | 7 | 8 | 9 | 10 |
| --- | --- | --- | --- | --- | --- |
| B | C | N | O | F | Ne |
| 15 | 14 | 15 | 16 | 17 | 18 |
| Al | Si | P | S | Cl | Ar |
| 31 | 32 | 33 | 34 | 35 | 36 |
| Ga | Ge | As | Se | Br | Kr |
| 49 | 50 | 51 | 52 | 53 | 54 |
| In | Sn | Sb | Te | I | Xe |
| 81 | 82 | 83 | 84 | 85 | 86 |
| Tl | Pb | Bi | Po | At | Rn |
| 113 | 114 | 115 | 116 | 117 | 118 |
| Nh | Fl | Mc | Lv | Ts | Og |
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I'll solve this problem by analyzing the periodic table trends for atomic radius.
Step 2: Understanding Atomic Radius Trends
Atomic radius varies systematically across the periodic table: - Down a group (column), atomic radius increases - Across a period (row), atomic radius generally decreases
Final Answer
Cs (Cesium) has the largest atomic radius in this periodic table.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students