QQuestionChemistry
QuestionChemistry
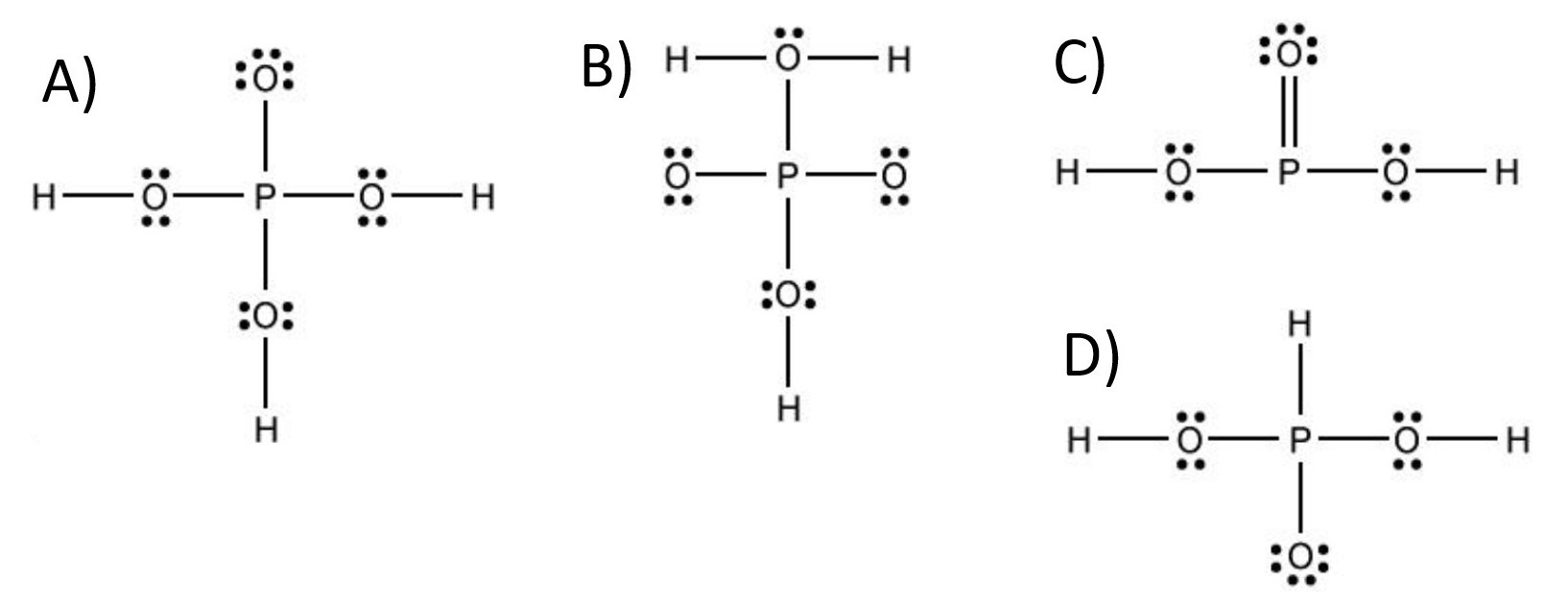
Which is the Lewis structure for H^3PO^4?
B) $H \square \ddot{0} \square H$
C) $\ddot{0}$ :
D)
D) $\quad \ddot{H}$
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Determine the total number of valence electrons in the Lewis structure for H^3PO^4.
5 (P) + 3 (H) = 8 \text{ valence electrons}
Phosphorus (P) is in the 5th period and group VA of the periodic table, so it has 5 valence electrons. Each hydrogen (H) atom has 1 valence electron. Therefore, the total number of valence electrons in H^3PO^4 is:
Step 2: Draw the skeletal structure of H^3PO^4.
Phosphorus is the central atom, and four sites are occupied by hydrogen atoms or single bonds. The skeletal structure is: ``` H - P - O | H | H ```
Final Answer
The closest correct option is D'), which includes the double-bonded oxygen atom with two lone pairs of electrons.
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students