QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
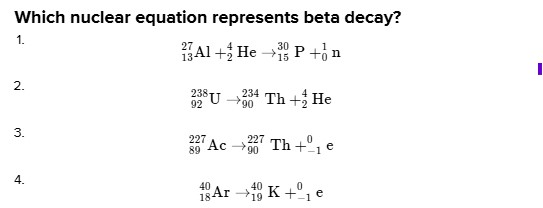
# Which nuclear equation represents beta decay?
1. $\quad{ }_{13}^{27} \mathrm{Al}+{ }_{2}^{4} \mathrm{He} \rightarrow{ }_{15}^{30} \mathrm{P}+{ }_{0}^{1} \mathrm{n}$
2. $\quad{ }_{92}^{238} \mathrm{U} \rightarrow{ }_{90}^{234} \mathrm{Th}+{ }_{2}^{4} \mathrm{He}$
3. $\quad{ }_{89}^{227} \mathrm{Ac} \rightarrow{ }_{90}^{227} \mathrm{Th}+{ }_{- 1}^{0} \mathrm{e}$
4. $\quad{ }_{18}^{40} \mathrm{Ar} \rightarrow{ }_{19}^{40} \mathrm{~K}+{ }_{- 1}^{0} \mathrm{e}$
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify the possible beta decay equation
where $${}_{-1}^{0} e$$ is the electron and $\bar{v}$ is the antineutrino.
Beta decay is a type of radioactive decay where a neutron is converted into a proton, an electron, and an antineutrino. This process can be represented by the nuclear equation: _{0}^{1} n \rightarrow{} _{1}^{1} p + {} _{- 1}^{0} e + \bar{v}
Step 2: Analyze the given nuclear equations
Let's analyze the given nuclear equations and see which one represents beta decay. Option 1: _{13}^{27} \mathrm{Al}+{ }_{2}^{4} \mathrm{He} \rightarrow{ }_{15}^{30} \mathrm{P}+{ }_{0}^{1} \mathrm{n} This is not a beta decay equation, as it involves the fusion of Aluminum- 27 and Helium- 4 to form Phosphorus- 30 and a neutron. Option 2: _{92}^{238} \mathrm{U} \rightarrow{ }_{90}^{234} \mathrm{Th}+{ }_{2}^{4} \mathrm{He} This is an alpha decay equation, where Uranium- 238 decays into Thorium- 234 and an alpha particle (Helium- 4 nucleus). Option 3: _{89}^{227} \mathrm{Ac} \rightarrow{ }_{90}^{227} \mathrm{Th}+{ }_{- 1}^{0} \mathrm{e} This is a beta decay equation, where Actinium- 227 decays into Thorium- 227 and an electron. Option 4: _{18}^{40} \mathrm{Ar} \rightarrow{ }_{19}^{40} \mathrm{~K}+{ }_{- 1}^{0} \mathrm{e} This is a beta decay equation, where Argon- 40 decays into Potassium- 40 and an electron.
Final Answer
The nuclear equations that represent beta decay are options 3 and 4: 1. $\quad{ }_{89}^{227} \mathrm{Ac} \rightarrow{ }_{90}^{227} \mathrm{Th}+{ }_{- 1}^{0} \mathrm{e}$ 2. $\quad{ }_{18}^{40} \mathrm{Ar} \rightarrow{ }_{19}^{40} \mathrm{~K}+{ }_{- 1}^{0} \mathrm{e}$
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students