QQuestionAnatomy and Physiology
QuestionAnatomy and Physiology
Which of the changes are chemical changes?
$\square$ Candle wax is melted.
$\square$ A candle is burned.
$\square$ A silver teapot turns black.
$\square$ Two clear colorless salt solutions are mixed and a bright orange precipitate forms.
$\square$ Sugar crystals are ground to a fine powder.
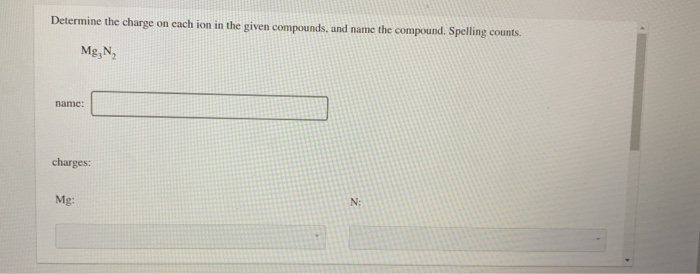
Determine the charge on each ion in the given compounds, and name the compound. Spelling counts.
\mathrm{Mg}_{3} \mathrm{~N}_{2}
name:
charges:
Mg :
$\mathrm{N}:$
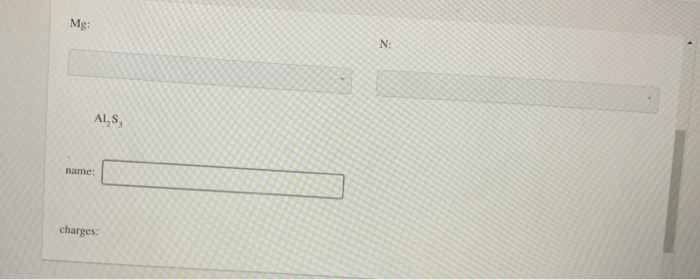
Mg:
N:
$\mathrm{Al}_{2} \mathrm{~S}_{3}$
name:
charges:
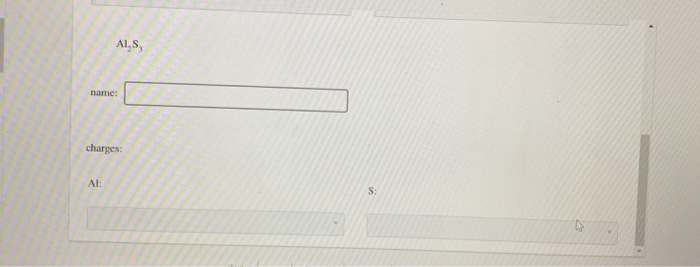
\mathrm{Al}_{2} \mathrm{~S}_{3}
name: $\square$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
$\qquad$
Consider the neutralization reaction
2 \mathrm{HNO}_{3}(\mathrm{aq})+\mathrm{Ba}(\mathrm{OH})_{2}(\mathrm{aq}) \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\mathrm{Ba}\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{aq})
A $0.110 -\mathrm{L}$ sample of an unknown $\mathrm{HNO}_{3}$ solution required 50.1 mL of $0.200 \mathrm{M} \mathrm{Ba}(\mathrm{OH})_{2}$ for complete neutralization. What is the concentration of the $\mathrm{HNO}_{3}$ solution?
concentration:
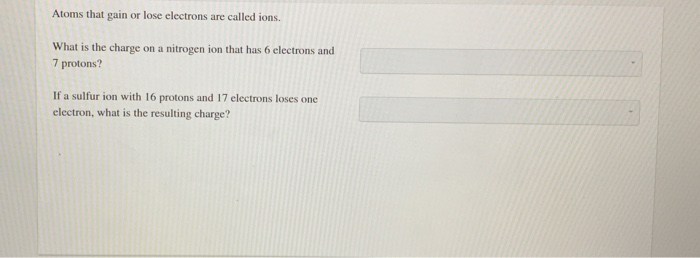
Atoms that gain or lose electrons are called ions.
What is the charge on a nitrogen ion that has 6 electrons and 7 protons?
If a sulfur ion with 16 protons and 17 electrons loses one electron, what is the resulting charge?
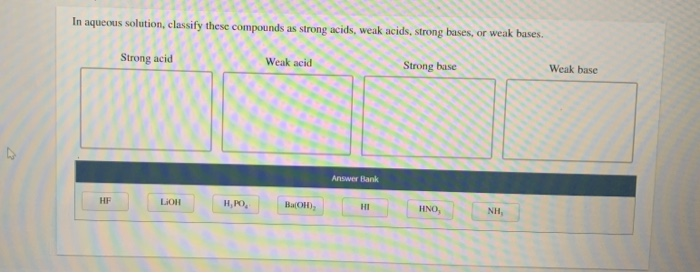
In aqueous solution, classify these compounds as strong acids, weak acids, strong bases, or weak bases.
Strong acid Weak acid Strong base Weak base
| | | | | | | |
| --- | --- | --- | --- | --- | --- | --- |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Attachments


6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1: Identify chemical changes
A chemical change occurs when new substances are formed. This involves a change in chemical properties and cannot be reversed by simple physical methods.
Step 2: Analyze the given options
- Candle wax is melted: This is a physical change since the wax only changes its state, not its chemical composition. - A candle is burned: This is a chemical change as new substances, such as carbon dioxide and water, are formed. - A silver teapot turns black: This is a chemical change due to tarnishing, which results from a reaction between silver and sulfur-containing substances in the air. - Two clear colorless salt solutions are mixed and a bright orange precipitate forms: This is a chemical change since a new substance (the precipitate) is formed. - Sugar crystals are ground to a fine powder: This is a physical change as the sugar's physical form changes but not its chemical composition.
Final Answer
The chemical changes are: - A candle is burned. - A silver teapot turns black. - Two clear colorless salt solutions are mixed and a bright orange precipitate forms. Step 4: Determine charges on ions and name compounds For \mathrm{Mg}_{3} \mathrm{~N}_{2}: Step 5: Identify charges and name - Mg: Since magnesium (Mg) has an atomic number of 12, it loses 2 electrons to achieve a stable electron configuration, resulting in a + 2 charge. - N: Nitrogen (N) has an atomic number of 7 and needs 3 more electrons to achieve a stable electron configuration, resulting in a - 3 charge. - Name: Magnesium nitride Step 6: Determine charges and name - Al: Aluminum (Al) has an atomic number of 13, so it loses 3 electrons to achieve a stable electron configuration, resulting in a + 3 charge. - S: Sulfur (S) has an atomic number of 16 and needs 2 more electrons to achieve a stable electron configuration, resulting in a - 2 charge. - Name: Aluminum sulfide - Charges: - Mg: + 2 - N: - 3 - Al: + 3 - S: - 2 - Names: - Magnesium nitride - Aluminum sulfide Step 8: Calculate the concentration of the \mathrm{HNO}_{3} solution We know that: - Volume of \mathrm{HNO}_{3} solution: 0.110 L - Volume of \mathrm{Ba(OH)}_{2} solution: 50.1 mL = 0.0501 L - Molarity of \mathrm{Ba(OH)}_{2} solution: 0.200 M Since one mole of \mathrm{Ba(OH)}_{2} reacts with two moles of \mathrm{HNO}_{3}, we have: Moles of \mathrm{HNO}_{3} = \frac{1}{2} \times \text{moles of } \mathrm{Ba(OH)}_{2} Using the molarity formula: Molarity = moles / volume We can find the moles of \mathrm{Ba(OH)}_{2}: Moles of \mathrm{Ba(OH)}_{2} = \text{molarity} \times \text{volume} = 0.200 \frac{\text{mol}}{\text{L}} \times 0.0501 \text{ L} = 0.01002 \text{ mol} Now, we can find the moles of \mathrm{HNO}_{3}: Moles of \mathrm{HNO}_{3} = \frac{1}{2} \times 0.01002 \text{ mol} = 0.00501 \text{ mol} Finally, we can calculate the concentration of the \mathrm{HNO}_{3} solution: Concentration = moles / volume = 0.00501 \text{ mol} / 0.110 \text{ L} = 0.0455 \frac{\text{mol}}{\text{L}} The concentration of the \mathrm{HNO}_{3} solution is 0.0455 M. Step 10: Analyze the charge of ions For a nitrogen ion with 6 electrons and 7 protons: - Since nitrogen has 7 protons, it has a + 7 charge (as it lacks 6 electrons). For a sulfur ion with 16 protons and 17 electrons: - If it loses one electron, its charge becomes + 16 + (- 1) = + 15. - Nitrogen ion charge: + 7 - Sulfur ion charge: + 15 (after losing one electron)
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students