QQuestionBiology
QuestionBiology
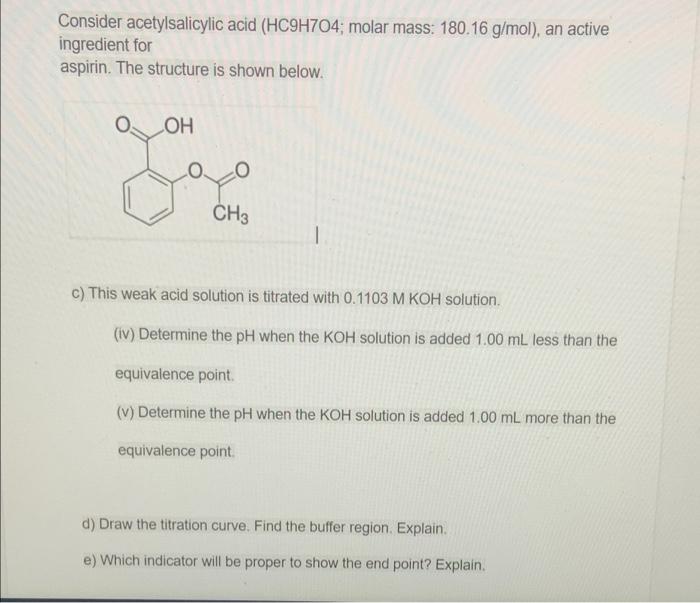
Consider acetylsalicylic acid (HC^9H^7O^4; molar mass: 180.16 g/mol), an active ingredient for aspirin. The structure is shown below.
c) This weak acid solution is titrated with 0.1103 M KOH solution.
(iv) Determine the pH when the KOH solution is added 1.00 mL less than the equivalence point.
(v) Determine the pH when the KOH solution is added 1.00 mL more than the equivalence point.
d) Draw the titration curve. Find the buffer region. Explain.
e) Which indicator will be proper to show the end point? Explain.
Attachments
6 months agoReport content
Answer
Full Solution Locked
Sign in to view the complete step-by-step solution and unlock all study resources.
Step 1I will solve parts (iv), (v), and d) of your problem.
pH = 14 - pOH = 14 - 2.24 \approx 11.76
Part (c)(iv) asks to determine the pH when 1.00 mL of KOH solution is added less than the equivalence point. Since we do not know the volume of KOH needed for the equivalence point, let's first determine the equivalence point. The balanced chemical equation for the titration is: The molar ratio between acetylsalicylic acid and KOH is 1:1. The initial moles of acetylsalicylic acid are: Let x be the moles of KOH added. Then, the moles of acetylsalicylic acid remaining after adding x moles of KOH are: The moles of KOH in excess are: At the equivalence point, all acetylsalicylic acid is neutralized, so: Now, let's consider adding 1.00 mL less than the equivalence point, i.e., adding 0.99 mL of KOH solution. The moles of KOH added are: The moles of acetylsalicylic acid remaining are: The moles of KOH in excess are: The total moles of dissociated ions are: Calculate the pOH: Calculate the pH: For part (c)(v), determine the pH when the KOH solution is added 1.00 mL more than the equivalence point. After adding 0.00111 L of KOH (1.00 mL), the moles of KOH added are: Now, there is an excess of hydroxide ions, and the solution is basic. The moles of hydroxide ions are: Calculate the pOH: Calculate the pH: For part (d), the titration curve and buffer region. The buffer region is the part of the titration curve where adding a strong base causes a small pH change. This region occurs before the equivalence point when a small amount of base is added and reacts with the weak acid, forming its conjugate base. In this case, the buffer region is from approximately 0 mL to 0.00555 L of KOH added. For part (e), the proper indicator. An ideal indicator for this titration should have a color change in the pH range of 4 - 6 (weak acid) or 8 - 10 (conjugate base). A suitable indicator would be phenolphthalein, which changes color from pink to colorless around pH 8 - 10. This indicator change will clearly show the endpoint of the titration.
Final Answer
pH = 14 - pOH = 14 - 2.24 \approx 11.76
Need Help with Homework?
Stuck on a difficult problem? We've got you covered:
- Post your question or upload an image
- Get instant step-by-step solutions
- Learn from our AI and community of students