Page 1
Loading page ...
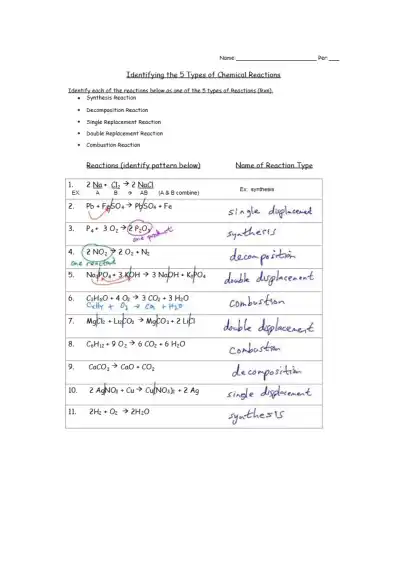
Master the 5 types of chemical reactions—synthesis, decomposition, single replacement, double replacement, and combustion—with examples and practice problems. Great for middle and high school chemistry review.

Loading page ...
This document has 3 pages. Sign in to access the full document!