A-level Chemistry: 3.3.11 Amines
This flashcard series focuses on naming various aliphatic amines and classifying them according to their type (primary, secondary, or tertiary). Each card prompts the learner to recognize the structure and correctly identify both the name and type of the amine.
Name the aliphatic amine and state the type of amine it is
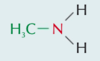
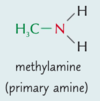
Key Terms
Name the aliphatic amine and state the type of amine it is
Name the aliphatic amine and state the type of amine it is
Name the aliphatic amine and state the type of amine it is
Name the aliphatic amine and state the type of amine it is
Name the aromatic amine and state the type of amine it is
Explain how a quaternary ammonium ion forms
Lone pair of electrons on nitrogen atom in tertiary amine can also bond with 4th organic group
Related Flashcard Decks
Study Tips
- Press F to enter focus mode for distraction-free studying
- Review cards regularly to improve retention
- Try to recall the answer before flipping the card
- Share this deck with friends to study together
| Term | Definition |
|---|---|
Name the aliphatic amine and state the type of amine it is | |
Name the aliphatic amine and state the type of amine it is | |
Name the aliphatic amine and state the type of amine it is | |
Name the aliphatic amine and state the type of amine it is | |
Name the aromatic amine and state the type of amine it is | |
Explain how a quaternary ammonium ion forms | Lone pair of electrons on nitrogen atom in tertiary amine can also bond with 4th organic group |
Explain how quaternary ammonium salts form | Quaternary ammonium ions are positively charged ∴ form bonds around with -ve ions & form complexes (quaternary ammonium salts) |
What type of quaternary ammonium salts are used as cationic surfactants? | Quaternary ammonium salts with at least 1 long hydrocarbon chain |
What products are cationic surfactants used in? | Fabric cleaners (e.g. fabric conditioner) and hair products (e.g. conditioner) |
Describe and explain why cationic surfactants are useful in fabric cleaners and hair products |
|
| ∵ accept protons |
Explain how amines can accept protons | Lone pair of electrons on nitrogen atom can form dative covalent (coordinate) bond with H+ ion |
What does the strength of a base depend on (for amines)? | Depends on how available nitrogen’s lone pair of electrons is More available the lone pair is = more likely amine is to accept proton & stronger base it’ll be |
When is a lone pair of electrons more available? | When its electron density is high |
Order the amines from the weakest to strongest base Ammonia, aliphatic amines, aromatic amines |
|
Explain why primary aromatic amines (e.g. phenylamine) act as very weak bases |
|
Explain why primary aliphatic amines act as strong bases |
|
Why are amines considered nucleophiles? | ∵ have lone pair of electrons |
Name the 2 mechanisms that amines partake in. Include the reactants. |
|
Name 2 ways you can make aliphatic amines |
|
Draw the mechanism to show how ethylamine can be made by reacting ammonia with bromoethane | |
When heating halogenoalkane with ammonia, what mixture of products do you get? | Mixture of primary, secondary, tertiary amines + quaternary ammonium salts |
Explain how heating halogenoalkane with ammonia results in a mixture of primary, secondary, tertiary amines and quaternary ammonium salts |
|
Write an equation for when a primary amine acts as a nucleophile with CH2CH2Br | Mechanism is similar to reaction of ammonia + halogenoalkane: 2 amine molecules react with halogenoalkane in succession to form more substituted amine (e.g. primary amine forms secondary amine) & ammonium salt with similar structure to original amine |
Describe 2 ways you can reduce a nitrile to a primary amine |
|
Why is the LiAlH4 method good in a lab but not good for industrial use? | LiAlH4 is too expensive | (industry uses catalytic hydrogenation) |
State how you can produce aromatic amines | Reduce nitro compound e.g. nitrobenzene |
Describe how you can produce an aromatic amine by reducing nitro compound (e.g. nitrobenzene) |
|
Write an equation representing the reduction of nitrobenzene into an aromatic amines | |
Give an example of how aromatic amines are useful compounds in organic synthesis | Are starting molecules for lots of dyes and pharmaceuticals |
Amides are _ ___ derivates | Amides are carboxylic acid derivates |
State the functional group of amides | -CONH2 |
Why do amides behave differently from amines? | Carbonyl group pulls electrons away from NH2 group |
Draw an amide | |
Draw an N-substituted amide | |
Define the inductive effect | Ability of alkyl group to release electrons towards nitrogen atom |
Give the formula of an organic compound that forms an alkaline buffer solution when added to a solution of ethylamine | CH3CH2NH3Cl
|
N-ethylpropanamide | |
Write an ionic equation for the reaction between propylamine and hydrochloric acid | CH3CH2CH2NH2 + H+ → CH3CH2CH2NH3+ (original equation: CH3CH2CH2NH2 + HCl → CH3CH2CH2NH3Cl) |
Propylamine reacts with bromoethane under specific conditions, to give a compound with the formula C9H22NBr. Draw the structure of the compound with the formula C9H22NBr. |