ACS Gen Chem 2 Final Exam Study Guide
This set of flashcards covers key topics in electrochemistry and nuclear chemistry, including electroplating calculations using Faraday’s laws, concentration cell voltage determination with the Nernst equation, identification of particles in nuclear reactions, and understanding the effects of beta decay on atomic number and mass.
Where is the hydrophilic (attracted to water) region of the molecule?
(A) Region 1
(B) Region 2
(C) Region 3
(D) The three regions are equally hydrophilic
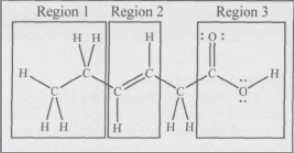
C
Key Terms
Where is the hydrophilic (attracted to water) region of the molecule?
(A) Region 1
(B) Region 2
(C) Region 3
(D) The three regions are equally hydrophilic
C
Which molecule is most soluble in water?
C
A solution of NaCl in water has a concentration of 20.5% by mass. What is the molal concentration of the solution?
Molar Mass NaCl = 58.44 g/mol
(A) 0.205 m
(B) 0.258 m
(C) 3.51 m
(D) 4.41 m
D
What is the mole fraction of water in 200 g of 89% (by mass) ethanol, C2H5OH?
Molar Mass C2H3OH = 46 g/mol
(A) 0.11
(B) 0.24
(C) 0.32
(D) 0.76
B
A mixture of 100 g of K2Cr2O7 and 200 g of water is stirred at 60 degrees C until no more of the salt dissolves. The resulting solution is poured off into a separate beaker, leaving the undissolved solid behind. The solution is now colled to 20 degrees C. What mass of K2Cr2O7 crystallizes from the solution during the cooling?
(A) 9 g
(B) 18 g
(C) 31 g
(D) 62 g
D
Carbonated beverages have a fizz because of gas dissolved in the solution. What increases the concentration of gas in a solution?
(A) cooling the solution
(B) heating the solution
(C) increasing the volume of solution
(D) decreasing the volume of solution
A
Related Flashcard Decks
Study Tips
- Press F to enter focus mode for distraction-free studying
- Review cards regularly to improve retention
- Try to recall the answer before flipping the card
- Share this deck with friends to study together
| Term | Definition |
|---|---|
Where is the hydrophilic (attracted to water) region of the molecule? | C |
Which molecule is most soluble in water? | C |
A solution of NaCl in water has a concentration of 20.5% by mass. What is the molal concentration of the solution? | D |
What is the mole fraction of water in 200 g of 89% (by mass) ethanol, C2H5OH? | B |
A mixture of 100 g of K2Cr2O7 and 200 g of water is stirred at 60 degrees C until no more of the salt dissolves. The resulting solution is poured off into a separate beaker, leaving the undissolved solid behind. The solution is now colled to 20 degrees C. What mass of K2Cr2O7 crystallizes from the solution during the cooling? | D |
Carbonated beverages have a fizz because of gas dissolved in the solution. What increases the concentration of gas in a solution? | A |
When 100 g of an unknown compound was dissolved in 1.00 kg of water, the freezing point was lowered by 6.36 degrees C. What is the identity of this unknown compound? | D |
Which 0.1 molal aqueous solution will have the lowest freezing point? | A |
What is the rate law of this reaction? | B |
The rate law for the reaction | B |
The half-life for the first order conversion of cyclobutene to ethylene, | C |
The half-life for the first-order radioactive decay of 32P is 14.3 days. How many days would be required for a sample of a radio pharmaceutical containing 32P to decrease to 20.0% of its initial activity? | A |
Plots are shown for the reaction N02 (g) --> NO (g) + 1/2 O2 (g) | D |
What changes when a catalyst is added to the reaction described by this energy diagram? | A |
Which statement regarding chemical reactions is true according to collision theory? | C |
The activation energy for a particular reaction is 83.1 kJ/mol. By what factor will the rate constant increase when the temperature is increased from 550.0 degrees C to 60.0 degrees C? | B |
Consider the reaction. | C |
Consider this equilibrium: | A |
What is the equilibrium expression for this reaction? | A |
Given the reaction and equilibrium constant: | C |
Consider the equilibrium reactions: | A |
Consider the reaction: | B |
Phosgene decomposes into carbon monoxide and elemental chlorine. If the initial concentration of COCl2 (g) is 0.50 M, what is the equilibrium concentration of CO (g)? | A |
BrCl (g) is in equilibrium with Br2 (g) and Cl2 (g) at 25 degrees C: | C |
Which will drive the equlibrium to form more Cu (s)? | B |
Which set represents a conjugate acid/base pair? | B |
What is the pH of a 0.820 M aqueous NH3 solution? | C |
Which acid is the weakest in aqueous solution? | C |
Which anion is the most basic? | A |
What is Kb of F-? | D |
Which substance will dissolve in water to produce an acidic solution? | A |
An acetate buffer contains equal volumes of 0.35 M HC2H3O2 | C |
What volume (in mL) of 0.150 M NaOH (aq) is required to neutralize 25.0 mL of 0.100 M H2SO4 (aq)? | B |
What is the Ksp expression for MgCO3 in water? | C |
Given the two reactions: | C |
What is the solubility product constant, Ksp, of Mg(OH)2 if its molar solubility in water is 1.6 x 10^-4 mol/L? | D |
Which compound has the highest molar solubility? | B |
A solution contains 0.002 M Pb^2+ and 0.002 M ag^+. What happens when NaCl (s) is added to bring the chloride ion concentration to 0.01 M? | D |
A sample of "hard" water contains about 2.0 x 10^-2 mol of Ca^2+ ions per liter. What is the maximum concentration of fluoride ion that could be present in hard water? Assume fluoride is the only anion present that will precipitate calcium ion. | C |
What will change the value of Ksp for silver azide (AgN3)? | D |
When comparing Q and Ksp of an unsaturated solution, _______. | A |
Of the four listed compounds, three have correct values for molar entropy, | A |
Which change is likely to be accompanied by an increase in entropy? | C |
For this reaction at 25 degrees C, | A |
When NH4NO3 dissolves in water, the temperature of the solution decreases. What describes the enthalpy and entropy changes of the system and which change drives the process? | D |
What is the free energy change for the formation of one mole of ammonia from its elements under standard conditions? | C |
Calculate the standard Gibbs free energy change at 298 K for the reaction: | B |
An equilibrium mixture of I2 (g), Cl2 (g), and ICl (g) at 298 K has partial pressures oof 0.0100 atm, 0.0100 atm, and 0.0900 atm, respectively. What is Delta G` at 298 K for the reaction: | B |
Which statement is correct for the reaction below under standard conditions? | B |
For a reaction where K > 1 at all temperatures, which statement(s) must be true? | C |
What is the coefficient for the nitrate ion (NO2^-) when the redox reaction is balanced in basic solution? | C |
A spontaneous electrochemical cell is set up as shown. Which statement is true? | C |
Given the standard reduction potentials at 25 degrees C, what is the standard cell potential of a | B |
An oxidation-reduction reaction in which 3 electrons are transferred has a Delta | B |
Consider the reaction. | B |
What half-reaction occurs at the annode during the electrolysis of molten sodium iodide? | A |
How many minutes are required to plate 2.08 g of copper at a constant flow of 1.26 A? | B |
For the concentration cell | B |
What is the missing particle in the nuclear equation? | B |
C | |
An atom of the element of atomic number 53 and mass number 131 undergoes beta decay. The residual atom after this change has an atomic number of _____ and a mass number of _____. | A |